Health
A branch of the flu family tree has died and won't be included in future US vaccines
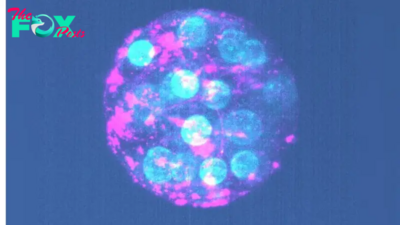
A type of flu virus that used to sicken people every year hasn't been spotted anywhere on Earth since March 2020. As such, experts have advised that the apparently extinct viruses be removed from next year's flu vaccines.
The now-extinct viruses were a branch of the influenza B family tree known as the Yamagata lineage. Scientists first reported the apparent disappearance of Yamagata viruses in 2021. At that time, experts speculated that precautions taken to stop the spread of COVID-19 — such as masking and social distancing — had not only driven the overall number of flu cases to historic lows but may have completely snuffed out this type of flu virus.
Now, according to news reports, a panel of advisers to the Food and Drug Administration (FDA) has unanimously agreed that Yamagata viruses should be dropped from the flu shot formulation for the 2024-2025 flu season. For the past decade, U.S. flu vaccines have protected against four types of flu — two iNFLuenza A strains and two iNFLuenza B strains — but that number will now fall to three.
The advisory committee has been pushing for this change for some time, STAT reported, and in fall 2023, the World Health Organization raised the same recommendation on a global scale.
Related: Is it too late to get a flu shot?
Dropping Yamagata from flu shot formulations could help boost manufacturers' production capacity, so they can make more doses. Plus, it would eliminate any potential risks associated with growing the virus in a lab — a process currently required to make flu vaccines, CNN reported.
And at baseline, experts emphasized that people needn't be vaccinated for something that appears to be extinct, STAT reported.
"We don't want to vaccinate you for a virus that's no longer in circulation for three, four years now," Dr. Hana El Sahly, a professor of molecular virology and microbiology at Baylor College of Medicine and chair of the FDA advisory committee, said at a meeting Tuesday (March 5).
"We've been talking about this for four years," Dr. Paul Offit, director of the Vaccine Education Center at Children's Hospital of Philadelphia and a member of the FDA advisory committee, told CNN.
—Scientists reveal rare antibodies that target 'dark side' of flu virus
—Skeletons from 1918 flu dispel myth that young, healthy adults were more vulnerable to the virus
—Could we ever eradicate the flu?
Despite the consensus among health officials, leaders in the pharmaceutical industry argued that manufacturers would need more time to switch to a trivalent formulation. Making the switch requires manufacturers to clear various regulatory hurdles. But with the FDA advisers pushing ahead, manufacturers are prepared to make trivalent vaccines for the U.S. starting this upcoming season; other countries will likely follow later, in accordance with their regulatory policies, STAT reported.
According to CNN, Jerry Weir, director of the FDA's Division of Viral Products, confirmed at the meeting Tuesday that all U.S. flu shot manufacturers have submitted the required regulatory paperwork and should be on track to make trivalent vaccines next season.
As these changes take effect, scientists around the world will continue to watch out for Yamagata alongside other flu viruses that infect people.
Ever wonder why some people build muscle more easily than others or why freckles come out in the sun? Send us your questions about how the human body works to community@liveScience.com with the subject line "Health Desk Q," and you may see your question answered on the website!
-

 Health11h ago
Health11h agoTeens Are Stuck on Their Screens. Here’s How to Protect Them
-

 Health17h ago
Health17h agoHow Pulmonary Rehab Can Help Improve Asthma Symptoms
-

 Health17h ago
Health17h ago10 Things to Say When Someone Asks Why You’re Still Single
-
 Health1d ago
Health1d agoThe Surprising Benefits of Talking Out Loud to Yourself
-

 Health1d ago
Health1d agoDoctor’s bills often come with sticker shock for patients − but health insurance could be reinvented to provide costs upfront
-

 Health2d ago
Health2d agoHow Colorado is trying to make the High Line Canal a place for everyone — not just the wealthy
-

 Health2d ago
Health2d agoWhat an HPV Diagnosis Really Means
-

 Health2d ago
Health2d agoThere’s an E. Coli Outbreak in Organic Carrots