Health
For some people, their genes and their cancer drugs don’t mix. A Colorado center is trying to fix that.
You have, right now inside you, a gene known as UGT1A1. But that’s so formal. Let’s just call it Eugene.
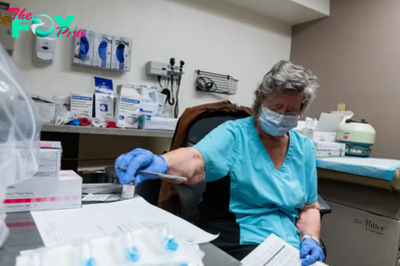
Eugene holds instructions for making enzymes that help your body break down certain substances, like bilirubin. This is great because it helps prevent babies from developing severe jaundice. But Eugene also works on other molecules, including the chemotherapy drug irinotecan.
Now, Eugene isn’t the same in everybody; it has a lot of different variations. One of those variations, known as *28, causes people to metabolize these substances more slowly. And that’s a problem for doctors trying to correctly dose irinotecan.
“If you have UGT1A1 *28, you are a very slow metabolizer,” said Dr. Wells Messersmith, a medical oncologist at UCHealth and an associate director at the University of Colorado Cancer Center. “If we give you a normal dose, you are going to get very sick.”
“In other words, it’s like an inadvertent overdose,” he said.
In the old days, doctors had to prescribe a dose and wait to see if bad things happened before adjusting. But this is where cutting-edge medicine comes in.
In connection with the Colorado Center for Personalized Medicine, certain cancer patients at UCHealth now undergo genetic screening at the start of their treatment. With the patient’s consent, these results then go into the center’s biobank, which links up with UCHealth’s electronic Health record system.
When a doctor goes to prescribe irinotecan to someone with a funky variant of Eugene, an alert pops up letting the doctor know of the genetic risk and recommending a different dose.
The Center for Personalized Medicine has around 230,000 patients in its biobank, and it recently crossed the 50,000 mark for patients receiving clinically relevant genetic results.
“Everybody always says, ‘This is so cool, it’s the future of medicine,’” said Dr. Christina Aquilante, the director of pharmacogenomics at the Center for Personalized Medicine. “It’s not the future. It’s the now. It’s happening.”
☀️ READ MORE
Colorado construction workers, pressured by longer, harder hours, die by suicide twice as often as other professions
Colorado’s medical aid-in-dying residency requirement won’t be lifted
Colorado Medicaid is discriminating against people with disabilities, federal complaint claims
The Center for Personalized Medicine is a collaboration between the University of Colorado School of Medicine, UCHealth and Children’s Hospital Colorado. It was formed in 2014 and began enrolling patients into the biobank the following year.
The hope is that the collaboration can serve a number of purposes, from improving patient care to also providing new data for research on the links between genetic variation and health conditions. In addition to the work identifying gene-drug interactions, results uploaded to the biobank can also help spot if a patient has a rare gene-driven condition before it’s too late.

Aquilante and others who work with the center say it is a national leader in integrating genetic testing and research with clinical patient care.
“There’s very few programs doing it just like us,” Aquilante said.
The center so far delivers results for seven genes that can affect how the body reacts to prescription drugs. They all have drug-gene links that are well documented in scientific research, Aquilante said.
Messersmith, the oncologist, said the hope is to find more. But the road to doing so may be tougher.

Isolating an individual gene’s impact on a drug is complicated, and Messersmith said, at least for cancer care, there currently aren’t other candidates where the connection is super clear. Meanwhile, acting on less-than-conclusive information could have consequences, as well.
“If we are too careful, we might dose-reduce too many people and thereby underdose people,” he said.
But CU officials are optimistic. Casey Greene, the interim director of the Center for Personalized Medicine, said the center’s work, in addition to benefiting researchers and patients, has the potential to reduce health care spending by reducing hospitalizations and other medical care related to bad drug-gene interactions.
-
 Health4h ago
Health4h agoThe Surprising Benefits of Talking Out Loud to Yourself
-

 Health6h ago
Health6h agoDoctor’s bills often come with sticker shock for patients − but health insurance could be reinvented to provide costs upfront
-

 Health21h ago
Health21h agoWhat an HPV Diagnosis Really Means
-

 Health1d ago
Health1d agoThere’s an E. Coli Outbreak in Organic Carrots
-

 Health2d ago
Health2d agoCOVID-19’s Surprising Effect on Cancer
-

 Health2d ago
Health2d agoWhat to Know About How Lupus Affects Weight
-

 Health5d ago
Health5d agoPeople Aren’t Sure About Having Kids. She Helps Them Decide
-

 Health5d ago
Health5d agoFYI: People Don’t Like When You Abbreviate Texts