Health
Tiny lab-grown testicles look remarkably like the real thing under the microscope
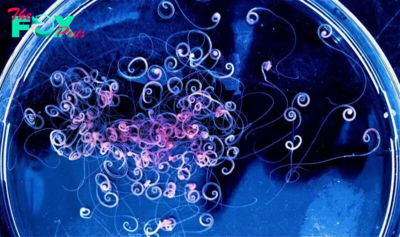
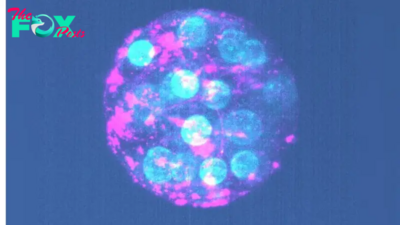
For the first time, scientists have grown three-dimensional, miniature versions of testicles in the lab, using mouse cells.
The lab-grown testicles survived in a dish for up to nine weeks and closely resembled natural mouse testicles, even developing tubelike structures equivalent to those that produce sperm in the testes of mice and humans. The cells within the model testicles also expressed genes similar to those that are active in regular mouse testicles.
The replica testicles are so-called organoids — self-organizing 3D structures that are grown to resemble full-size tissues in the body. Scientists have created organoids of many organs, including the human heart and brain, with the aim of better capturing the complexities of these tissues in 3D and in a way that represents human biology better than animal models do. Organoids are usually created by coaxing stem cells to develop into tissue-like structures by exposing them to specialized growth chemicals in a dish.
The researchers behind the new testicle organoids described their achievement in a paper published Jan. 12 in the International Journal of Biological Sciences. They say the organoids could help improve our understanding of the testicles, or testes, whose main job is to produce the male sex hormone testosterone and sperm needed for reproduction. As such, the organoids could also provide valuable insight into conditions that are linked to improper testicle functioning, such as sex development disorders and male infertility, the scientists say.
Related: In a 1st, 'minibrains' grown from fetal brain tissue
"Once we have an in vitro [petri dish] cellular model of the testis, we can start exploring how the testes function, how different cell types within the testis interact with each other, and also try to explore whether sperm can be generated in vitro," Nitzan Gonen, co-senior study author and a researcher in biomedicine at Bar-Ilan University in Israel, told Live Science in an email.
Gonen and colleagues study a process called sex determination, whereby either male or female reproductive organs — the testicles and ovaries, respectively — form during embryonic development. The researchers were inspired to create the new testicle organoids after realizing there was a lack of lab models that closely resembled these organs in the human body.
In the new study, the team extracted immature testicle cells from newborn mice and, with the help of growth-stimulating signaling proteins, drove them to form organoids. The organoid cells organized themselves in a similar way to what is seen in a normal mouse testicle.
The team was also able to grow testicle organoids from mouse testicle cells drawn from developing embryos. Many diseases that are tied to testicle development and dysfunction occur during embryonic development, so being able to model this stage of testicle growth may be particularly important, the team wrote in the paper.
—Scientists develop 'crying' model of human eye tissue
—'Mini placentas' may reveal roots of pregnancy disorders like preeclampsia
—In a 1st, scientists combine AI with a 'minibrain' to make hybrid computer
The newly made testicle organoids didn't produce sperm. However, there were early signs that the cells might be able to take part in meiosis, a type of cell division that is used to produce various sex cells, including sperm. Going forward, the team wants to explore whether the organoids can be made to make sperm and hormones.
They also aspire to grow testicle organoids from human cells, and they already have one specific use in mind. Male children with cancer who are undergoing chemotherapy often lose the stem cells that will go on to form sperm after puberty begins and can, therefore, become infertile, Gonen said. The hope is that, eventually, testicle organoids could be grown from a child's stem cells before cancer treatment begins and be used to produce fertile sperm, she said. These sperm could then be available for future fertility treatments, such as in vitro fertilization (IVF.)
Ever wonder why some people build muscle more easily than others or why freckles come out in the sun? Send us your questions about how the human body works to community@liveScience.com with the subject line "Health Desk Q," and you may see your question answered on the website!
-
 Health13h ago
Health13h agoThe Surprising Benefits of Talking Out Loud to Yourself
-

 Health15h ago
Health15h agoDoctor’s bills often come with sticker shock for patients − but health insurance could be reinvented to provide costs upfront
-

 Health21h ago
Health21h agoHow Colorado is trying to make the High Line Canal a place for everyone — not just the wealthy
-

 Health1d ago
Health1d agoWhat an HPV Diagnosis Really Means
-

 Health1d ago
Health1d agoThere’s an E. Coli Outbreak in Organic Carrots
-

 Health2d ago
Health2d agoCOVID-19’s Surprising Effect on Cancer
-

 Health2d ago
Health2d agoColorado’s pioneering psychedelic program gets final tweaks as state plans to launch next year
-

 Health3d ago
Health3d agoWhat to Know About How Lupus Affects Weight