Health
New self-swab HPV test is an alternative to Pap smears. Here's how it works.
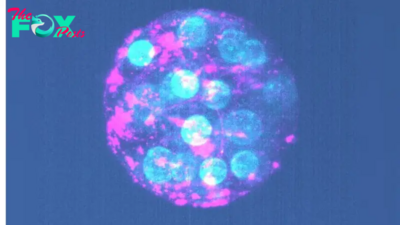
A new option for cervical cancer screening gives patients a less-invasive alternative to conventional tests.
These new "self-collection tests" are scheduled to arrive in doctor's offices nationwide this month. The Food and Drug Administration (FDA) approved self-collection as a method to detect human papillomavirus (HPV), the leading cause of cervical cancer, in May. Screening tests are intended to flag people at high risk of cancer or precancer, not to diagnose the disease.
This FDA approval enables patients to collect their own clinical samples for cervical cancer screening. With the rollout of these tests, the U.S. joins Australia, Canada, the Netherlands, Denmark and Sweden, where self-swabbing for HPV is already widely used.
For now, the samples, collected from the vaginal canal, must still be gathered in Health care settings, such as doctor's offices. Other countries have allowed at-home self-sampling for HPV, but the method is still pending FDA approval in the U.S.
Here's what you should know about the newly available self-collection tests.
How is HPV related to cervical cancer?
HPV is a common sexually transmitted infection that's primarily transferred through sexual intercourse or skin-to-skin contact. Most sexually active people will contract at least one type of HPV in their lifetime, but the infection normally resolves on its own.
Though more than 30 types of HPV can infect the genitals, only a small number — referred to as "high-risk" HPV — are associated with cancer. Low-risk HPV tends to have no symptoms and clears on its own, although sometimes, genital warts may appear. Nonetheless, this low-risk type of infection rarely leads to cancer.
-
 Health2h ago
Health2h agoThe Surprising Benefits of Talking Out Loud to Yourself
-

 Health3h ago
Health3h agoDoctor’s bills often come with sticker shock for patients − but health insurance could be reinvented to provide costs upfront
-

 Health9h ago
Health9h agoHow Colorado is trying to make the High Line Canal a place for everyone — not just the wealthy
-

 Health19h ago
Health19h agoWhat an HPV Diagnosis Really Means
-

 Health1d ago
Health1d agoThere’s an E. Coli Outbreak in Organic Carrots
-

 Health1d ago
Health1d agoCOVID-19’s Surprising Effect on Cancer
-

 Health2d ago
Health2d agoColorado’s pioneering psychedelic program gets final tweaks as state plans to launch next year
-

 Health2d ago
Health2d agoWhat to Know About How Lupus Affects Weight