Health
Designer immune-cell therapy could shrink deadly brain tumors, early trials show
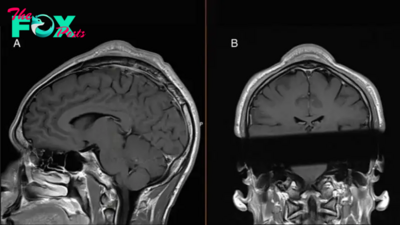
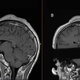
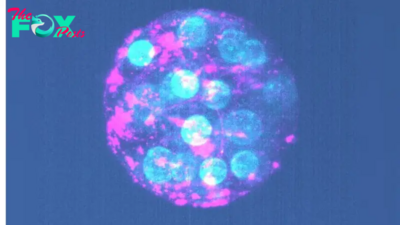
A new immune cell-based treatment for glioblastoma, the most aggressive type of brain cancer, has shown promise in shrinking tumors in the short-term, according to two early clinical trials.
The trials tested the safety and effectiveness of a personalized therapy called chimeric antigen receptor (CAR) T-cell therapy. This involves drawing out and genetically manipulating patients' immune cells, known as T cells, to more effectively recognize and attack tumors once they're reintroduced into the body.
One of the trials, described in a paper published Wednesday (March 13) in the New England Journal of Medicine (NEJM), included three patients with recurrent glioblastoma, meaning their cancer had returned following standard radiation and chemotherapy. T cells from these patients were genetically modified to target two versions of a receptor called epidermal growth factor receptor (EGFR) on the surface of tumor cells.
In the second trial, published the same day in the journal Nature Medicine, researchers used CAR T cells to target EGFR and an additional tumor-related receptor, called interleukin-13 receptor alpha 2, in six patients with recurrent glioblastoma.
Related: In a 1st, scientists use designer immune cells to send an autoimmune disease into remission
Both trials found that CART-cell therapy was safe and reduced tumor size in all nine patients. In the Nature Medicine study, patients saw these reductions within one or two days, while in the NEJM study they saw them after one to five days. One NEJM patient's tumor almost completely regressed five days after a single treatment, while another person's tumor decreased in size by 60.7% after 69 days.
However, these effects didn't necessarily last. Tumors returned for two patients in the NEJM study, within either 72 days or a month after the initial infusion. The other patient showed no signs of tumor recurrence more than 150 days after treatment, although this was the last assessment point of the study so it is uncertain whether this occurred afterward. Some of the reductions seen in the Nature Medicine study have also been sustained for several months, for example up to seven months in one patient.
-
 Health3h ago
Health3h agoThe Surprising Benefits of Talking Out Loud to Yourself
-
 Health5h ago
Health5h agoDoctor’s bills often come with sticker shock for patients − but health insurance could be reinvented to provide costs upfront
-

 Health11h ago
Health11h agoHow Colorado is trying to make the High Line Canal a place for everyone — not just the wealthy
-

 Health20h ago
Health20h agoWhat an HPV Diagnosis Really Means
-

 Health1d ago
Health1d agoThere’s an E. Coli Outbreak in Organic Carrots
-

 Health1d ago
Health1d agoCOVID-19’s Surprising Effect on Cancer
-

 Health2d ago
Health2d agoColorado’s pioneering psychedelic program gets final tweaks as state plans to launch next year
-

 Health2d ago
Health2d agoWhat to Know About How Lupus Affects Weight